Consider the following reaction: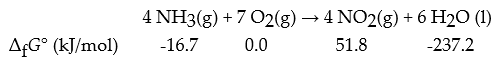
What is ΔrG° for this reaction in kJ?
Definitions:
Heat Energy
Energy that results from the random movement of atoms, ions, or molecules; the greater the amount of heat energy in an object, the higher the object’s temperature.
Reversible Reactions
Chemical reactions that can proceed in both the forward and backward directions under certain conditions, achieving a state of equilibrium.
Exchange Reaction
A chemical reaction where parts of two reacting molecules are interchanged to produce new products.
Synthesis Reaction
A process where multiple substances merge to create a compound that is more complex.
Q31: Which of the following mixtures would you
Q50: A sweetened cup of coffee is an
Q61: The iodine atom in I<sub>2</sub> would be
Q62: Choose the correct statement about a container
Q66: Calculate the total quantity of heat required
Q74: Which of the following pairs of liquids
Q76: 0.653 g of a monoprotic acid (MW=
Q79: For BeCl<sub>2</sub>,the dipole moment of the molecule,hybridization
Q83: The process in which a gas is
Q96: Phosphorus pentachloride decomposes to phosphorus trichloride at