In the Haber process,ammonia is synthesized from nitrogen and hydrogen: 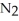 (g) +
(g) + 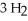 (g) →
(g) → 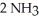 (g)
(g)
ΔrG ° at 298 K for this reaction is -33.3 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of 1.9 atm 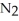 ,1.6 atm
,1.6 atm 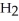 ,and 0.65 atm
,and 0.65 atm 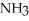 Is ________ kJ mol-1.
Is ________ kJ mol-1.
Definitions:
Amicable Discharge
A mutual agreement between an employer and an employee to end employment on good terms.
Employee Engagement
The degree to which employees are fully involved in their work and the strength of their commitment to their job and company.
Competitive Advantage
A unique attribute or ability a company has that enables it to outperform its competitors in the market.
Self-Evaluations
The process by which individuals assess their own job performance, strengths, and areas needing improvement, often part of performance management systems.
Q16: Which of the following atoms has the
Q18: What is the pH of a 0.30
Q22: Choose the INCORRECT statement.<br>A)Bonds between like atoms
Q28: Choose the INCORRECT statement.<br>A)The van't Hoff equation
Q56: What is the bond order for the
Q77: The fluorocarbon <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="The fluorocarbon
Q85: A reaction is spontaneous if:<br>I.ΔG is a
Q86: Determine the [C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup>] of the following aqueous
Q92: The compound ClF<sub> </sub> contains:<br>A)ionic bonds<br>B)nonpolar covalent
Q105: What is the formal charge on B