At 20 °C,an aqueous solution that is 24.0% by mass in ammonium chloride has a density of 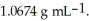 What is the molarity of ammonium chloride in the solution?
What is the molarity of ammonium chloride in the solution?
The formula weight of 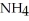 Cl is
Cl is 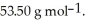
Definitions:
Mechanism
Describes the detailed steps of a chemical reaction, explaining how reactants are transformed into products at the molecular level.
Step-by-step
An approach or method that proceeds gradually from one clearly defined stage to the next.
Stereochemical
Pertaining to the spatial arrangement of atoms in molecules and the impact of this arrangement on their chemical behavior and reactions.
Regio-
A prefix used in chemistry to describe the orientation or position of functional groups within molecules.
Q16: For the molecule <br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="For the
Q32: What is the approximate concentration of free
Q42: What is the approximate pH at the
Q58: According to molecular orbital (MO)theory,which statement below
Q78: Consider an aqueous solution which is 0.020
Q81: Write the equilibrium constant expression for the
Q85: Which combination of hybrid orbital descriptions and
Q101: Which of the following compounds has the
Q116: In the titration of a solution of
Q128: What is the pH of a 1.0