Calculate the freezing point of a solution containing 5.0 grams of KCl and 550.0 grams of water.The molal freezing point depression constant ( 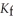 ) for water is
) for water is 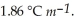
Definitions:
Inward
Refers to movements or directions towards the inside or center of something.
Expansionary Gap
A situation where the actual output of an economy exceeds its potential output at full employment, often leading to inflation.
Inflationary Pressure
The upward pressure on prices that may arise from factors such as increased demand or rising costs, leading to inflation.
Seasonal Unemployment
Unemployment resulting from seasonal variations in demand or supply of labor, often seen in industries like agriculture or tourism.
Q14: Choose the Br∅nsted-Lowry acids and bases in
Q19: As the activity a of a substances
Q41: A pH 4.88 buffer was prepared by
Q44: Two solutions contain the same amount of
Q44: In a reaction at equilibrium involving only
Q48: Given the data below,determine the molar enthalpy
Q58: To a concentrated buffer of pH 4.0
Q74: What is the [HPO<sub>4</sub><sup>-2</sup>] of a solution
Q83: Find correct statements.<br>I.A spontaneous process is a
Q99: What volume in mL of 0.05 M