Consider the following hypothetical equilibrium reaction:
A2(g) + B2(g) ⇌ 2 AB(g) where Kc = 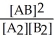
The equilibrium constant for the reaction: 2 A2(g) + 2 B2(g) ⇌ 4 AB(g) is ________.
Definitions:
Spot Rate
The current market price at which a currency can be bought or sold for immediate delivery and payment.
Forward Rate
A financial term describing the agreed-upon exchange rate for currencies to be exchanged at a future date between parties.
Foreign Exchange Gain
A profit arising from the increase in value of one currency against another.
Exchange Rates
The rate at which one currency can be exchanged for another, which can fluctuate based on market conditions.
Q1: Write the equilibrium constant expression for the
Q26: When the vapor pressure of a liquid
Q29: According to the molecular orbital (MO)theory,when two
Q34: What is the weight percent of vitamin
Q39: Choose the INCORRECT statement.<br>A)Electrons are transferred in
Q56: What is the cell diagram for the
Q60: A solution of LiCl in water is
Q76: Commercial perchloric acid is 70.0% by mass,HClO<sub>4</sub>(aq),and
Q94: The enthalpy change for converting 1.00 mol
Q108: For the reaction PCl<sub>5</sub>(g)→ PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g)at 298