Consider the equilibrium system: N2O4(g) ⇌ 2 NO2(g) for which Kp = 0.1134 at 25 °C and ΔrH° = 58.03 kJ/mol.Assuming that the total pressure inside the container is 10 atm at equilibrium and that initially only N2O4 was present inside the container,compute 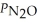
At equilibrium.
Definitions:
Period Cost
Expenses that are not directly tied to the production process and are charged to the accounting period in which they occur, like selling and administrative expenses.
Controllable Costs
Expenses that can be directly managed or influenced by a specific manager or management level within an organization.
Uncontrollable Costs
Expenses over which a manager or business has no direct control, often determined by external factors.
Production Manager
A professional responsible for overseeing the production process within a manufacturing facility, ensuring that goods are produced efficiently, safely, and meet quality standards.
Q3: What is the hydronium ion concentration of
Q3: Consider the following hypothetical equilibrium reaction:<br>A<sub>2</sub>(g)+ B<sub>2</sub>(g)⇌
Q40: For the production of NO<sub>2</sub>,K<sub>c</sub> = [NO<sub>2</sub>]<sup>2</sup>/[NO]<sup>2</sup>[O<sub>2</sub>].At
Q42: Gas solubility always increases with temperature.
Q46: For the decomposition of ammonium carbamate<br>NH<sub>4</sub>(NH<sub>2</sub>CO<sub>2</sub>)(s)⇌ 2
Q50: A solution with a hydrogen ion concentration
Q59: An azeotropic mixture is a:<br>A)mixture of two
Q79: What is the hydronium ion concentration in
Q97: In which of the following aqueous solutions
Q125: What is the [OH<sup>-</sup>] of a solution