Multiple Choice
What is the pH of a 0.500 mol L-1 NH3 aqueous solution that has Kb = 1.8 × 10-5?
The equation for the dissociation of NH3 is below: 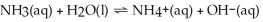
Definitions:
Related Questions
Q14: Standard Gibbs energy of formation requires the
Q25: The van't Hoff factor is due to
Q28: A concentrated buffer of pH 8.0 is
Q28: What is the pH of a 0.380
Q40: Write the net redox reaction that occurs
Q40: For the second-order reaction A → products,the
Q57: Equal volumes of 0.020 M Ag<sup>+</sup>(aq)solution and
Q86: If the rate of a specific chemical
Q100: For NaCl,predict whether the aqueous solution is
Q116: Which of the following is FALSE for