Multiple Choice
A concentrated buffer of pH 8.0 is added to an equal volume of an aqueous solution that is 0.080 M in each of the ions Zn2+,Ni2+,and Mn2+.The expected precipitate would consist of ________.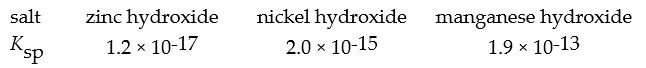
Identify how learning from experience shapes employee behavior and efficiency.
Distinguish between unconditioned and conditioned stimuli and responses in the learning process.
Understand how behaviors are reinforced in the workplace and the impact on employee performance.
Recognize the role of managers in shaping desirable behaviors through reinforcement and understanding of behavioral changes.
Definitions:
Related Questions
Q28: A concentrated buffer of pH 8.0 is
Q46: An aqueous solution has [HC<sub>7</sub>H<sub>5</sub>O<sub>2</sub>] = 0.100
Q50: Determine the pH of the following aqueous
Q63: In a nonmetal hydroxide the oxygen-hydrogen bond
Q65: A solute's molar solubility and its molarity
Q66: Determine the pH of the following aqueous
Q77: The difference between temporary hard water and
Q92: How many mL of 0.200 M aqueous
Q96: When equal volumes of the indicated aqueous
Q109: Determine the pH of the following aqueous