Calculate the pH of a 0.60 mol L-1 aqueous H2SO3 solution that has the stepwise dissociation constants Ka1 = 1.5 × 10-2 and 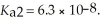
Definitions:
Tariffs
Taxes imposed by a government on imported goods and services to restrict imports or generate revenue.
Import Quotas
Restrictions set by a country on the quantity of goods that can be imported within a certain time frame to protect domestic industries.
Domestic Employment
The total number of people employed within a country's borders, reflecting the health of the economy and labor market.
Services Abroad
The provision of services by a country or business to clients or customers located in other countries.
Q20: What is the balanced chemical equation for
Q32: What is the pH of an aqueous
Q59: Determine the K<sub>sp </sub>at 25 °C of
Q60: At 900.0 K,the equilibrium constant (K<sub>p</sub>)for the
Q65: How will addition of sodium acetate to
Q84: The following reaction is exothermic.<br>2 N<sub>2</sub>O(g)→ 2
Q92: The isomerization of methylisonitrile to acetonitrile <br><img
Q98: A solution of nonvolatile solute has an
Q104: The solubility of a salt MX<sub>2</sub> with
Q122: Mixing comparable amounts of a strong acid