An aqueous solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 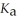
Of nitrous acid is 1.36 × 10-3.
Definitions:
Private Good
A product or service that is typically consumed by the purchaser and cannot be used or consumed by others.
Utility Function
A mathematical representation in economics depicting the relationship between consumption choices and levels of satisfaction or happiness.
Labatt Ale
A popular brand of beer produced in Canada, known for its history and variety of brews.
Single Peaked
A type of preference or opinion that, when graphed, shows a single top or highest preference, typically used in voting theory to describe voters' preferences over various issues.
Q1: How many seconds are required to produce
Q12: The solubilities of ammonium bromide,NH<sub>4</sub>Br,in water at
Q22: Complete the following reaction:<br>B(OH)<sub>3</sub>(aq)+ 2 H<sub>2</sub>O(l)→<br>A)B ∙
Q28: What is the mole fraction of oxygen
Q28: A concentrated buffer of pH 8.0 is
Q34: What is the concentration of the acetate
Q35: In the first-order,reaction A → products,[A] =
Q85: The common ion in an aqueous solution
Q115: It is easier to calculate the pH
Q123: For the reaction: C<sub>2</sub>H<sub>4</sub>Br<sub>2</sub> + 3 KI