A 25.0 mL sample of 0.150 mol L-1 aqueous hypochlorous acid is titrated with a 0.150 mol L-1 NaOH(aq) solution.What is the pH after 26.0 mL of base is added? The 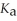
Of hypochlorous acid is 3.0 × 10-8.
Definitions:
Boxer Rebellion
An uprising in China (1899-1901) by the "Boxers" aiming to rid the country of foreign influence and Christian missions.
Buffalo Soldiers
Nickname for the African American soldiers who served in the U.S. Army's 10th Cavalry Regiment after the Civil War, playing a significant role in the American West.
Black Troops
African American soldiers, especially those who served in the United States armed forces during the Civil War and other conflicts, often facing segregation and discrimination.
Black Protests
Refers to demonstrations and movements by Black individuals and allies to fight against racial injustice, discrimination, and systemic racism.
Q3: Consider the following hypothetical equilibrium reaction:<br>A<sub>2</sub>(g)+ B<sub>2</sub>(g)⇌
Q26: What volume of an aqueous solution of
Q27: List the following acids in order of
Q35: For the reaction: 3 Fe(s)+ 4 H<sub>2</sub>O(g)⇌
Q50: A sweetened cup of coffee is an
Q64: Choose the INCORRECT completion of the following
Q64: Calculate the pH for an aqueous solution
Q65: For the reaction: 2 N<sub>2</sub>O<sub>5</sub>(g)→ 4 NO<sub>2</sub>(g)+
Q82: The pH of an aqueous solution at
Q112: The term pH = -ln [H<sup>+</sup>].