Calculate the standard enthalpy change for the reaction
2C8H18(l) + 17O2(g) 16CO(g) + 18H2O(l) .
Given: 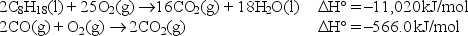
Definitions:
Preventable Hazard
A risk or danger to health and safety that can be minimized or eliminated through proactive measures, planning, and awareness.
Nonlinear Dose Response
A relationship in which the effect of exposure to a substance does not directly correlate to the dose received, often observed with toxins and pharmaceuticals.
Threshold Level
The point at which a relatively small change or variation in external conditions can cause a rapid change in an ecosystem or in the properties of a substance.
Technological System
An organized arrangement of people, machines, and procedures designed to perform and control various functions and processes for achieving specific objectives.
Q6: Which one of the following is most
Q37: Batteries in our cars generate electricity
Q43: Define electronegativity:<br>A)an atoms ability to attract electrons
Q46: Electrons in an orbital with l =
Q46: Determine the oxidation number of each of
Q67: The formal charge on the bromine atom
Q67: Define Charles's Law
Q74: Using the figure below, categorize electromagnetic radiation
Q105: The electron affinity of fluorine is essentially
Q132: A neon atom in its ground state