Use the Born-Haber cycle to calculate the standard enthalpy of formation ( H 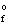 ) for LiCl(s) given the following data:
) for LiCl(s) given the following data:
H(sublimation) Li = 155.2 kJ/mol
I1 (Li) = 520 kJ/mol
Bond energy (Cl-Cl) = 242.7 kJ/mol
EA (Cl) = 349 kJ/mol
Lattice energy (LiCl(s) ) = 828 kJ/mol
Definitions:
Sternberg
Refers to Robert Sternberg, a psychologist known for his theory on intelligence and creativity, including the triarchic theory of intelligence.
Analytical
The ability to critically evaluate information or situations, often involving breaking down complex problems into smaller, more manageable parts.
Acquire Knowledge
The process of learning or gaining information, insights, or skills through study, experience, or instruction.
Intelligence
Intelligence refers to the capacity for learning, understanding, and applying knowledge, as well as the ability to adapt to new situations and solve problems.
Q11: The elements in Group 7A are known
Q24: Which one of the following elements is
Q47: A molecule with 3 single bonds (and
Q50: Identify the dominant (strongest)type of intermolecular force
Q60: A solution that is 33.6 % by
Q63: The electrons in the delocalized molecular orbitals
Q69: An aerosol can with a volume of
Q71: The scattering of helium atoms by a
Q101: Given the following <span class="ql-formula"
Q127: What phase exists at the point labeled