The isomerization of cyclopropane to propene follows first-order kinetics. 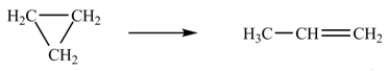 At 700 K, the rate constant for this reaction is 6.2 × 10-4 min-1.How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
At 700 K, the rate constant for this reaction is 6.2 × 10-4 min-1.How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene?
Definitions:
Neonates
Newborn infants, especially during the first few weeks after birth.
Uterine Contractions
Are the tightening and relaxing of the uterine muscles, often experienced during childbirth or menstruation.
Efface
To thin.
Dilate
To widen or enlarge.
Q5: The equilibrium constant for the chemical equation<br>2NO(g)+
Q13: Consider this gas phase equilibrium system:
Q33: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q69: For the reaction A + 3B
Q82: At a particular temperature the first-order
Q100: Calculate the activation energy, in kJ/mol,
Q100: The common allotropes of carbon (graphite
Q114: Dichloromethane, CH<sub>2</sub>Cl<sub>2</sub>, is an important solvent in
Q120: Write the Lewis structure for the product
Q128: A 8.0 M solution of formic acid