Consider this gas phase equilibrium system: PCl5(g) 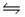
PCl3(g) + Cl2(g) Hºrxn = +87.8 kJ/mol.
Which of these statements is false?
Definitions:
Siemens Bribery Scandal
A major corruption case involving the German conglomerate Siemens AG, which was accused of using bribes to secure contracts around the world.
Enron Scandal
Refers to a major accounting fraud that led to the bankruptcy of the Enron Corporation, an energy, commodities, and services company, in 2001.
Cases
Instances or examples used for study, analysis, or reference in various academic or professional contexts.
Property Rights
Legal rights to use, control, and derive benefits from a property or resource.
Q47: Identify the conjugate acid-base pairs in the
Q54: Calculate the equilibrium constant K<sub>c</sub> for the
Q54: The heat of solution<br>A)is never positive
Q66: What is the pH of a solution
Q67: The vapor pressure of a solution depends
Q74: Equilibrium constants are known for the following
Q94: What volume of 0.0500 M sodium hydroxide
Q105: Which of the following is both a
Q111: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math
Q125: Arrange the acids HBr, H<sub>2</sub>Se, and H<sub>3</sub>As