The isomerization of cyclopropane to form propene is a first-order reaction. 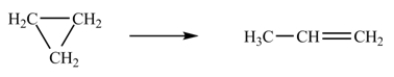 At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min.Determine the rate constant for this reaction at 760 K.
At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min.Determine the rate constant for this reaction at 760 K.
Definitions:
Q22: Given the following data for the reaction:
Q26: Arrange the following aqueous solutions in order
Q27: During osmosis<br>A)pure solvent diffuses through a membrane
Q38: Nitrous oxide (N<sub>2</sub>O)decomposes at 600°C according
Q48: According to VSEPR theory, which one of
Q48: Calculate the pH of the solution resulting
Q57: Identify the conjugate base of HSO<sub>4</sub><sup> -</sup><br>A)OH<sup>-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)H<sub>2</sub>O<br>D)H<sub>2</sub>SO<sub>3</sub><br>E)SO<sub>4</sub><sup>2-</sup>
Q67: Write an equation showing the net reaction
Q107: How many liters of ethylene glycol antifreeze
Q146: Write the formula for the conjugate acid