For the chemical reaction system described by the diagram below, which statement is true? 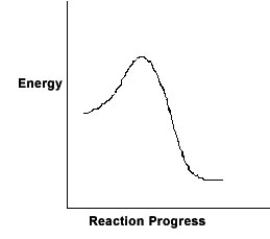
Definitions:
Canada Revenue Agency
The federal agency responsible for administering tax laws for the Canadian government and various provinces and territories, as well as overseeing various social and economic benefit and incentive programs through taxation.
Income Tax Return
A document filed with the government that reports income, expenses, and other pertinent tax information.
Depreciation Method
A systematic approach used to allocate the cost of a tangible asset over its useful life, reflecting consumption, wear and tear, or obsolescence of the asset.
Units-of-Production Method
A depreciation method where the expense is based on the asset's usage, output, or units produced, rather than time.
Q23: Of the following substances, KCl, KBr, and
Q43: All intermolecular forces must be overcome in
Q72: Predict the direction in which the equilibrium
Q72: The intermolecular forces present in HSCH<sub>2</sub>CH<sub>2</sub>SH include
Q73: When the substances in the equation
Q94: What volume of 0.0500 M sodium hydroxide
Q101: What is the molality of a solution
Q102: The solubility of silver chloride can be
Q121: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many
Q177: For maleic acid, HOOCCH=CHCOOH, K<sub>a1</sub> = 1.42