When the substances in the equation below are at equilibrium, at pressure P and temperature T, the equilibrium can be shifted to favor the products by CuO(s) + H2(g) 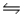
H2O(g) + Cu(s) Hºrxn = -2.0 kJ/mol
Definitions:
Mexican Foods
Culinary dishes and traditions originating from Mexico, characterized by flavors, ingredients like corn and beans, and dishes such as tacos and enchiladas.
External Secondary Data
Information that was collected for some purpose other than the problem at hand, found outside of the organization.
U.S. Households
The living units occupied by one or more persons in the United States, characterized by various demographics.
Packaging
The materials and methods used to protect, transport, and present goods for sale.
Q1: In the reaction, 2N<sub>2</sub>O <span
Q22: Given the following data for the reaction:
Q32: The rate determining step must be the
Q35: Under what conditions (always, never, high
Q49: Nitric oxide reacts with chlorine to
Q59: If 14.2 g of Al(NO<sub>3</sub>)<sub>3</sub> is dissolved
Q60: The salt hydrolysis reaction for NH<sub>4</sub>NO<sub>3</sub> that
Q68: Which of the following phase changes is
Q101: Under what conditions (always, never, high temperature
Q126: Calculate the pH of a buffer solution