On analysis, an equilibrium mixture for the reaction 2H2S(g) 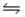 2H2(g) + S2(g) was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel.Calculate the equilibrium constant, Kc, for this reaction.
2H2(g) + S2(g) was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel.Calculate the equilibrium constant, Kc, for this reaction.
Definitions:
Loan Date
The specific date on which a loan agreement is signed, and the funds are disbursed to the borrower.
Plant Capacity
Plant capacity refers to the maximum output or production that a manufacturing facility can achieve under normal conditions.
Variable Costs
Costs that vary directly with the level of production or volume of output, in contrast to fixed costs.
Fixed Costs
Costs that remain constant regardless of a company's level of activity, including lease payments, wages, and insurance fees.
Q24: For the reaction at equilibrium 2SO<sub>3</sub>
Q24: Indicate all the types of intermolecular forces
Q32: The most space efficient arrangement of spheres
Q37: Which one of the following substances should
Q61: Rubidium has a heat of vaporization
Q65: Crystals of elemental sulfur are easily crushed,
Q67: For the reaction H<sub>2</sub>(g)+ S(s) <span
Q122: The number of atoms in a body-centered
Q127: What phase exists at the point labeled
Q149: Which of the following substances is