For the reaction at equilibrium 2SO3 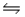 2SO2 + O2 ( Hºrxn= 198 kJ/mol) , increasing the reaction temperature would:
2SO2 + O2 ( Hºrxn= 198 kJ/mol) , increasing the reaction temperature would:
Definitions:
Return On Total Assets
A financial ratio that measures the profitability of a company relative to its total assets.
Year 2
This refers to the second year of a business or financial operation, often used in context to compare data year-over-year.
Earnings Per Share
A company's net profit divided by the number of its outstanding shares, indicating the portion of a company's profit allocated to each share of stock.
Dividend Yield Ratio
A financial metric that measures the dividend per share a company pays out to its shareholders relative to its share price, indicating the income return on the investment.
Q4: The normal boiling point of bromine is
Q17: In benzene (C<sub>6</sub>H<sub>6</sub>), what is the hybridization
Q49: NaCl is added slowly to a solution
Q65: Concerning the following reaction at equilibrium: 3Fe(s)+
Q80: Given the following K<sub>a</sub> values, which anion
Q85: Calculate the pH of a 6.7 ×
Q94: If one starts with pure NO<sub>2</sub>(g)at a
Q108: The salt hydrolysis reaction for CH<sub>3</sub>COONa that
Q137: What is the pH of a 0.30
Q141: Calculate the hydrogen ion concentration in a