Consider the reaction N2(g) + O2(g) 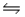 2NO(g) , for which Kc = 0.10 at 2,000ºC.Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
2NO(g) , for which Kc = 0.10 at 2,000ºC.Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
Definitions:
Competition
The rivalry among businesses to attract customers and achieve higher sales, profits, and market share.
Monopoly
A market structure characterized by a single seller offering a unique product or service without close substitutes, leading to control over pricing and market conditions.
Vertical Mergers
A business practice where companies operating at different stages of the production process for a specific good or service merge.
Concentration
The action or power of focusing all one's attention or mental effort on a particular object or activity.
Q5: Which one of the following substances is
Q15: Is the reaction SiO<sub>2</sub>(s)+ Pb(s) <span
Q19: Two moles of PCl<sub>5</sub> are placed in
Q22: What volume of 0.200 M potassium hydroxide
Q37: A 0.10 M HF solution is 8.4%
Q56: Due to a highway accident, 150 L
Q66: A reaction with an equilibrium constant K<sub>c</sub>
Q81: Methane has a heat of fusion of
Q116: The K<sub>sp</sub> for silver(I)phosphate is 1.8 ×
Q122: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img