Two moles of PCl5 are placed in a 5.0 L container.Dissociation takes place according to the equation PCl5(g) 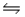
PCl3(g)+ Cl2(g).At equilibrium, 0.40 mol of Cl2 are present.Calculate the equilibrium constant (Kc)for this reaction under the conditions of this experiment.
Definitions:
Quantitative Data
A type of data that can be counted or measured and expressed numerically, facilitating statistical analysis.
Debt Servicing
The process of making interest and principal payments on debt over time.
Callable Bonds
Bonds that can be redeemed by the issuer before their maturity date at a predetermined price.
Market Interest Rates
The prevailing rates at which borrowers can obtain loans and lenders can lend in the money market, influenced by the central bank policies, supply and demand.
Q1: Determine the equilibrium constant (K<sub>eq</sub>)at 25°C for
Q11: The pOH of a solution is 10.40.Calculate
Q17: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)
Q56: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q69: What is the percent by mass of
Q79: Which of the following is consistent
Q96: Write a net ionic equation for the
Q102: Calculate the mass of solute in the
Q109: Calculate the percent ionization of formic acid
Q171: Morphine, C<sub>17</sub>H<sub>19</sub>NO<sub>3</sub>, is often used to control