Hydrogen iodide decomposes according to the equation 2HI(g) 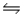 H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC.0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of H2 at equilibrium.
H2(g) + I2(g) , for which Kc = 0.0156 at 400ºC.0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC.Calculate the concentration of H2 at equilibrium.
Definitions:
Abraham Lincoln
The 16th President of the United States who served from 1861 to 1865 and led the country through the Civil War while abolishing slavery with the Emancipation Proclamation and the 13th Amendment.
Popular Sovereignty
The concept that a state and its government's power is established and maintained by the approval of its citizens, via their chosen representatives.
Slavery Abolition
The movement towards and the process of formally ending the legal practice of slavery and human enslavement in various parts of the world.
Tariff of Abominations
The nickname given to the Tariff of 1828 in the United States, which imposed high duties on imports and sparked significant controversy, especially in the South.
Q6: A certain reaction A <span
Q9: Sodium carbonate, Na<sub>2</sub>CO<sub>3</sub>(s), can be prepared by
Q15: Hard water deposits (calcium carbonate)have built up
Q47: The following initial rate data apply
Q59: The oxidation of iodide ions by
Q66: Which of the following gases is expected
Q96: Which is expected to have a higher
Q107: Hydrogen peroxide (H<sub>2</sub>O<sub>2</sub>)decomposes according to the
Q113: K<sub>w</sub> for the auto-ionization of water,
Q128: Which one of the following reactions