The Kc for the reaction CO2(g)+ H2(g) 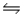
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of water in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Discount Factor
A multiplier used to calculate the present value of future cash flows, reflecting the time value of money and risk.
Invested Sum
The total amount of money put into a particular investment or project.
Discount Rate
The interest rate used to discount future cash flows to their present value, often used in capital budgeting and investment planning.
Lease Payments
Regular payments made by a lessee to a lessor for the use of an asset for a specific period of time.
Q20: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q22: What is the boiling point of a
Q27: Starting with 0.250L of a buffer solution
Q28: Arrange the following in order of increasing
Q33: In which one of the following solutions
Q53: Consider the following reaction: 2Fe<sup>2+</sup>(aq)+ Cu<sup>2+</sup>
Q55: The entropy of a perfectly ordered crystalline
Q79: Calculate the molar solubility of AgBr in
Q93: What is the freezing point of an
Q108: The normal freezing point of ammonia