A quantity of liquid methanol, CH3OH, is introduced into a rigid 3.00-L vessel, the vessel is sealed, and the temperature is raised to 500K.At this temperature, the methanol vaporizes and decomposes according to the reaction CH3OH(g) 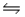
CO(g) + 2 H2(g) , Kc= 6.90×10-2.
If the concentration of H2 in the equilibrium mixture is 0.426M, what mass of methanol was initially introduced into the vessel?
Definitions:
Restraints of Trade
Legal or economic restrictions placed on the free exchange or movement of goods, services, or labor, often to maintain fair competition or prevent monopolistic practices.
Sherman Antitrust Act
A landmark federal statute in the U.S. passed in 1890 that prohibits monopolistic business practices and promotes competition in the marketplace.
Sherman Antitrust Act
A landmark U.S. law enacted in 1890 to counteract or reduce monopolies and maintain competition among businesses.
Interstate Commerce Commission
A regulatory agency in the United States established to oversee railroad rates and later expanded to other modes of transportation, until its operations were phased out in the late 20th century.
Q45: The reaction A + 2B
Q55: The following mechanism has been suggested
Q56: Which of the following statements is false?<br>A)A
Q58: It is possible for the following
Q64: In how many grams of water should
Q75: Chlorine dioxide reacts in basic water
Q100: Given the following data, estimate the
Q113: You have 500.0 mL of a buffer
Q114: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q119: The boiling points of propanol (CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>OH)and pentanol