Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below.
CaCO3(s) 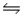
CaO(s)+ CO2(g)
The KP for this reaction is 1.16 at 800°C.A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C.Ignoring the volume occupied by the solid, what will be the overall mass percent of carbon in the solid once equilibrium is reached?
Definitions:
Dividends Paid
The portion of a company's earnings that is distributed to shareholders as a return on their investment.
Paid-in Capital
The total amount of money that shareholders have invested in a company through the purchase of its stock.
Treasury Stock
Shares that were issued and later reacquired by the issuing company, reducing the amount of outstanding stock on the open market.
Debit Balance
A debit balance is the amount of money that a business or individual owes; it reflects transactions that have been debited to an account, increasing the total debit.
Q9: What is the osmotic pressure of a
Q14: The atomic planes in a graphite crystal
Q16: What phase exists at the point labeled
Q45: Consider the weak bases below and their
Q54: For the reaction X<sub>2</sub> + Y
Q64: The following mechanism has been suggested
Q87: Will a precipitate form (yes or no)when
Q89: An endothermic solution process is described
Q104: The following reaction in aqueous solution
Q127: Calculate the pH of the solution resulting