The Kc for the reaction CO2(g)+ H2(g) 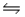
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of hydrogen gas in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Adverse Selection
A situation in markets where buyers and sellers have access to different information, leading to transactions where one party may be at a disadvantage, often discussed in the context of insurance and used cars.
Individual Mandate
A requirement that individuals purchase health insurance or pay a penalty, aimed at ensuring universal coverage in health care systems.
Health Insurance Market
The marketplace where individuals, families, and employers purchase health insurance coverage.
Long-term Care Insurance
Insurance coverage designed to cover the costs of long-term care services, including both medical and non-medical needs for people with a chronic illness or disability.
Q14: The vapor pressure of a solution depends
Q19: What molar ratio of benzoate ion to
Q40: What conditions are used in the Haber
Q42: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q54: Which one of the following substances should
Q60: At 30°C, by how much is a
Q72: Predict the direction in which the equilibrium
Q81: Methane has a heat of fusion of
Q102: Calculate the pH of a 0.20 M
Q153: The overall reaction 2Co<sup>3+</sup>(aq)+ 2Cl<sup>-</sup>(aq) <span