Calculate Kp for the reaction 2NOCl(g) 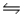 2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 × 10-2.
2NO(g) + Cl2(g) at 400°C if Kc at 400°C for this reaction is 2.1 × 10-2.
Definitions:
Upregulation
The process of increasing the quantity or activity of a molecular component, often referring to gene expression or protein levels in cell biology.
Downregulation
The process by which a cell decreases the quantity of a cellular component, such as RNA or protein, in response to an external variable.
Withdrawal
The process or experience of stopping or reducing the use of a psychoactive substance, often leading to uncomfortable symptoms.
ATP
Adenosine triphosphate, a molecule that stores and transfers energy within cells, enabling metabolic processes to take place.
Q6: Determine the equilibrium constant K<sub>p</sub> at
Q17: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)
Q28: For the reaction HCONH<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q28: N,N-diethyl-m-tolumide (DEET)is the active ingredient in many
Q35: Which of the following compounds is more
Q53: Calculate the minimum concentration of Mg<sup>2+</sup> that
Q62: What is the optimum pH of a
Q77: A liquid boils when its<br>A)vapor pressure is
Q105: In general, to calculate the rate constant
Q163: Which one of these equations represents