On analysis, an equilibrium mixture for the reaction 2H2S(g) 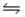 2H2(g) + S2(g) was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel.Calculate the equilibrium constant, Kc, for this reaction.
2H2(g) + S2(g) was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel.Calculate the equilibrium constant, Kc, for this reaction.
Definitions:
Hypothermia
A medical emergency that occurs when the body loses heat faster than it can produce heat, causing a dangerously low body temperature.
Hyperthermia
An abnormally high body temperature, often resulting from external factors like heat exposure or internal disease processes.
Hypoglycemia
A condition where blood sugar (glucose) levels are lower than normal, which can cause symptoms like shakiness, sweating, and confusion.
Feeding Intolerance
Difficulty in digesting food without adverse effects, often seen in infants and those with digestive system disorders.
Q35: Under what conditions (always, never, high
Q42: What is the molar mass of toluene
Q46: Calculate the pH of the solution resulting
Q54: Calculate the equilibrium constant K<sub>c</sub> for the
Q55: A solution of chloroform, CHCl<sub>3</sub>, and acetone,
Q57: Find the temperature at which K<sub>p</sub>
Q62: What is the optimum pH of a
Q78: Which would have the higher boiling point
Q85: Consider this reaction at equilibrium at a
Q116: The K<sub>sp</sub> for silver(I)phosphate is 1.8 ×