Consider this reaction at equilibrium at a total pressure P1: 2SO2(g) + O2(g) 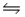
2SO3(g)
Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished.The new equilibrium total pressure will be
Definitions:
Factory Overhead
Indirect costs associated with manufacturing, including costs related to operating the manufacturing facilities, aside from direct labor and direct materials costs.
Machine Hours
A measure of the amount of time a machine is operated, used in allocating manufacturing costs.
Journal Entry
A record in accounting that shows a business transaction and its effect on the financial statements, consisting of debit and credit entries.
Predetermined Overhead Rate
A method employed to distribute manufacturing overhead costs to specific production units, utilizing projected expenses and levels of activity.
Q8: Which liquid is expected to have the
Q26: The solubility product for chromium(III)fluoride is K<sub>sp</sub>
Q57: Find the temperature at which K<sub>p</sub>
Q70: List the following solutions in order of
Q71: What is the molarity of a solution
Q73: HCN is classified as a weak acid
Q79: The K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g)
Q85: Assuming equal concentrations of conjugate base and
Q96: Write a net ionic equation for the
Q178: Calculate the pH of a 1.6 M