Consider the reaction N2(g) + O2(g) 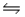 2NO(g) , for which Kc = 0.10 at 2,000ºC.Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
2NO(g) , for which Kc = 0.10 at 2,000ºC.Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
Definitions:
Transfer of Exclusive Possession
The act of transferring the right to exclusively occupy or use property or goods to another party.
Car
A wheeled motor vehicle designed for the transportation of people, typically having four tires and powered by an internal combustion engine or electric motor.
Malfunction
The failure of a device, system, or process to operate as intended.
Duty of Care
A legal obligation to adhere to a certain standard of reasonable care while performing acts that could foreseeably harm others.
Q15: Indicate the number of <span
Q15: At a certain temperature, the data
Q31: The K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g)
Q33: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q36: At a certain temperature, the data
Q73: The vapor pressure of water at 45.0
Q76: Calculate the pH of the solution resulting
Q96: Calculate the pH of a 0.021 M
Q115: Which of the following compounds is more
Q133: If the pH of an acid rain