The Kc for the reaction CO2(g)+ H2(g) 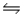
H2O(g)+ CO(g)is 1.6 at about 990ºC.Calculate the number of moles of water in the final equilibrium system obtained by initially adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00 L reactor at 990ºC.
Definitions:
Cyclical Unemployment
This type of unemployment is caused by the downturns in the business cycle, where workers lose their jobs due to a lack of demand during economic recessions.
Discouraged Worker
An individual who has stopped looking for employment due to the belief that no jobs are available for them or there are none for which they would qualify.
BLS
The Bureau of Labor Statistics, a US government agency responsible for collecting and analyzing critical economic data, including employment and inflation.
Natural Unemployment Rate
The lowest level of unemployment that an economy can sustain over the long term without causing inflation.
Q2: A solution was prepared such that the
Q72: The intermolecular forces present in HSCH<sub>2</sub>CH<sub>2</sub>SH include
Q84: Which is the correct equilibrium constant expression
Q92: The activation energy for the reaction
Q100: Indicate all the types of intermolecular forces
Q102: Predict the signs (-, +, or
Q114: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q121: With respect to the figure below, which
Q122: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img
Q140: The molar enthalpy of vaporization of carbon