For the following reaction at equilibrium in a reaction vessel, which change will cause the Br2 concentration to decrease? 2NOBr(g) 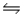
2NO(g) + Br2(g) , Hºrxn= 30 kJ/mol
Definitions:
Availability Heuristic
A cognitive bias that causes people to overestimate the likelihood of events based on their ability to recall similar instances.
Escalation Heuristic
A cognitive bias or decision-making process where individuals increase their commitment to a previously taken course of action beyond the level that is justified by the current situation.
Satisficing Heuristic
A decision-making strategy that involves searching through the available alternatives until an acceptability threshold is met, rather than seeking the best possible solution.
Design Thinking
An iterative process in solving problems creatively and innovatively by understanding the human needs involved, re-framing the problem in human-centric ways, and adopting a hands-on approach in prototyping and testing.
Q3: What is the molarity of a solution
Q19: What molar ratio of benzoate ion to
Q23: Which of the following would be expected
Q27: Which one of the following substances crystallizes
Q35: An aqueous dextrose solution having a density
Q76: The intermolecular forces present in CH<sub>3</sub>NH<sub>2</sub> include
Q99: Which one of these equations represents
Q103: Which one of the following substances should
Q106: Will a precipitate of magnesium fluoride form
Q121: With respect to the figure below, which