Concerning the following reaction at equilibrium: 3Fe(s) + 4H2O(g) 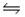
Fe3O4(s) + 4H2(g) , increasing the concentration of the Fe(s) would:
Definitions:
Cardiac Arrhythmia
An abnormal heart rhythm that can include conditions where the heartbeat is too slow, too fast, or irregular.
Hypostatic Pneumonia
A type of pneumonia that occurs from prolonged immobility, leading to the pooling and congestion of secretions in the lungs.
Captopril
A medication used to treat high blood pressure, heart failure, and certain types of kidney problems by relaxing blood vessels and making it easier for the heart to pump blood.
Ductus Arteriosus
A blood vessel in fetuses that bypasses lung circulation by connecting the pulmonary artery directly to the ascending aorta; it normally closes after birth.
Q13: Consider this gas phase equilibrium system:
Q23: Hydrogen iodide decomposes according to the equation:<br>2HI(g)
Q30: The N - N - H bond
Q38: Which one of the following equations represents
Q50: Calculate the silver ion concentration in a
Q64: The pH at the equivalence point of
Q66: Which of the following gases is expected
Q80: For the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q106: Potassium bromide, KBr, crystallizes like NaCl in
Q106: The oxides SO<sub>2</sub> and N<sub>2</sub>O<sub>5</sub> will form