Equilibrium constants are known for the following reactions:
S(s)+ 3/2O2(g) 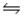
SO3(g)Kc = 9.2 × 1023
SO3(g) 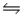
SO2(g)+ 1/2O2(g)Kc = 4.8 × 10- 4
Thus, for the reaction S(s)+ O2(g) 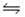
SO2(g), Kc = 4.4 × 1020.
Definitions:
Navigation Slides
Noncontent slides that tell your audience where you’re going and where you’ve been.
Department Affiliation
The association or connection of an individual or entity with a specific department within an organization.
Company Affiliation
The connection or association of an individual with a particular business or organization, often as an employee or partner.
Emotional Tone
The underlying feeling or mood conveyed by an author or speaker through their choice of words and style of communication.
Q29: Identify the dominant (strongest)type of intermolecular force
Q40: For the reaction BrO<sub>3</sub><sup>- </sup>+ 5Br<sup>-</sup>+
Q46: Consider the reaction N<sub>2</sub>(g)+ O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q49: The pOH of a solution is 9.60.Calculate
Q56: Indicate all the types of intermolecular forces
Q61: What is the pH of 10.0 mL
Q84: Suppose the atoms in a two-dimensional crystal
Q99: Given that the heat of vaporization of
Q111: Suppose the atoms in a two-dimensional crystal
Q123: Which one of these salts will form