Multiple Choice
Consider this reaction at equilibrium: 2SO2(g) + O2(g) 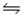
2SO3(g) , Hºrxn = -198 kJ/mol
If the volume of the system is compressed at constant temperature, what change will occur in the position of the equilibrium?
Definitions:
Related Questions
Q15: For the nitrogen fixation reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g)
Q17: The peroxodisulfate ion can oxidize iodide
Q17: Consider the equilibrium equation C(s)+ H<sub>2</sub>O(g)
Q24: A sample of solid naphthalene is introduced
Q34: The first-order decomposition, A <span
Q75: The geometry of the hybrid orbitals of
Q77: Calculate the pH at the equivalence point
Q95: Which of these species would you expect
Q106: Which of the following is consistent
Q130: In the following picture, each arrow represents