A quantity of liquid methanol, CH3OH, is introduced into a rigid 3.00-L vessel, the vessel is sealed, and the temperature is raised to 500K.At this temperature, the methanol vaporizes and decomposes according to the reaction CH3OH(g) 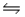
CO(g) + 2 H2(g) , Kc= 6.90×10-2.
If the concentration of H2 in the equilibrium mixture is 0.426M, what mass of methanol was initially introduced into the vessel?
Definitions:
Society View
The collective attitudes, perspectives, and norms held by a society or community about various issues and entities.
Public Rituals
Ceremonial acts or events conducted in the public eye that are often symbolic, celebrating or commemorating social or cultural values and traditions.
Durkheim
A foundational French sociologist best known for his theories on social structures, collective conscience, and the study of suicide.
Society Bond
The connection or relationship individuals have with their community or society, often influencing their social behaviors and attitudes.
Q6: Consider this reaction at equilibrium: 2SO<sub>2</sub>(g)+
Q44: Calculate the H<sup>+</sup> ion concentration in a
Q64: In how many grams of water should
Q69: For the reaction A + 3B
Q95: What is the mole fraction of NaOH
Q104: In which of the following solvents would
Q110: Based on the phase diagram shown below,
Q111: For the hypothetical reaction A +
Q139: Solid iodine has a vapor pressure of
Q166: Al(OH)<sub>3</sub> is an amphoteric hydroxide.Write a balanced