Consider the equilibrium C(s)+ H2O(g) 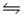
CO(g)+ H2(g), H = 2296 J.What will happen to the concentration of carbon if gaseous water is added to the system?
Definitions:
Decline Stage
The phase in a product's life cycle characterized by a decrease in sales and profitability, indicating the product is nearing the end of its market life.
Maturity Stage
A phase in the product life cycle where growth is diminished, and sales are steady or declining.
Test Marketing
The practice of testing new products in a controlled, limited market prior to a full-scale launch.
Competitors
Entities that are in the same industry or market and compete against each other for customers, market share, or resources.
Q12: Hydrogen iodide decomposes according to the equation:<br>2HI(g)
Q44: The specific heat of liquid ethanol, C<sub>2</sub>H<sub>5</sub>OH(l),
Q51: Consider the equilibrium C(s)+ H<sub>2</sub>O(g) <img
Q53: For the following exothermic reaction, the
Q70: A sample of rainwater collected near a
Q74: A saturated sodium carbonate solution at 100°C
Q101: Which would have the stronger intermolecular forces
Q142: The oxides CO<sub>2</sub> and SO<sub>3</sub> will form
Q144: What is the pH of a 0.15
Q170: When comparing acid strength of binary acids