A 0.10 M NH3 solution is 1.3% ionized.Calculate the H+ ion concentration. NH3 + H2O 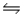
NH4+ + OH-
Definitions:
Control Population
A group in an experimental study that does not receive the treatment and is used as a benchmark to measure how the other tested groups compare.
Significance Level
The threshold below which an observed result is considered statistically significant, usually denoted by alpha.
Sample Data
Data collected from a portion of a larger population, used to make inferences about the whole population.
P-Value
The p-value is a measure in statistical hypothesis testing representing the probability of obtaining a result at least as extreme as the observed one, assuming the null hypothesis is true.
Q14: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q24: A sample of solid naphthalene is introduced
Q56: Which of the following aqueous solutions has
Q66: A reaction with an equilibrium constant K<sub>c</sub>
Q68: Ammonium ion (NH<sub>4</sub><sup>+</sup>)reacts with nitrite ion (NO<sub>2</sub><sup>-</sup>)to
Q74: Calculate the boiling point of a 4.5
Q83: The solubility of strontium carbonate is 0.0011
Q91: Which one of these net ionic
Q94: Concerning the rate law, Rate = k[A]<sup>0</sup>,
Q98: Given the following K<sub>a</sub> values, which anion