For the reaction CuS(s)+ H2(g) 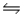
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 798 K and 1 atm pressure.
Definitions:
Flex-Time
Flex-Time is an employment practice that allows workers to choose their work hours within a specified range to accommodate personal needs and preferences, promoting work-life balance.
Compressed Workweek
A work schedule that allows an employee to work a full-time job in fewer days than the traditional five-day workweek.
Flexible Work Arrangements
Employment terms that allow employees to vary their working hours, locations, or schedules to integrate work with personal life effectively.
Prenatal Viral Infections
Viruses contracted by the mother during pregnancy that can cross the placental barrier and potentially harm the developing fetus.
Q10: Consider the reaction N<sub>2</sub>(g)+ 3H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q27: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q36: If the reaction 2H<sub>2</sub>S(g)<sub> </sub> <sub> </sub>
Q38: Consider a Galvanic cell constructed from
Q56: What effect does increasing temperature have on
Q67: The solubility of silver bromide can be
Q97: As a result of alpha emission, the
Q102: A metal object is to be gold-plated
Q131: For the electrochemical cell, Cd(s)| Cd<sup>2+</sup>(aq)|| Co<sup>2+</sup>(aq)|
Q133: Predict the products of the electrolysis of