Predict the direction in which the equilibrium will lie for the reaction H3PO4(aq) + HSO4-(aq) 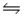
H2PO4-(aq) + H2SO4(aq) .
Ka1(H3PO4) = 7.5 × 10-3; Ka(H2SO4) = very large
Definitions:
Racial Passing
The act of a person classified as a member of one racial group being accepted or identifying as a member of another, often to escape racial discrimination.
White Ethnics
Refers to individuals of European descent, other than those of Anglo-Saxon background, who maintain cultural practices, values, or ways of life associated with their heritage.
Italian Americans
Americans of Italian descent, who are part of the United States' ethnic and cultural fabric, contributing to its diversity, history, and society.
Irish Americans
People in the United States who have full or partial ancestry from Ireland, often reflecting a rich cultural heritage and history of immigration.
Q5: Which of the following processes would
Q12: Given that the heat of vaporization of
Q21: How many grams of water are needed
Q28: An electrochemical cell based on the
Q44: Which of these metals will reduce water
Q57: Consider the following reactions and their associated
Q70: The free energy of formation of nitric
Q80: Dissolving an ionic solid in water always
Q83: For the reaction SbCl<sub>5</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q86: What is the pH of a solution