Predict the direction in which the equilibrium will lie for the reaction H2SO3(aq) + HCO3- (aq) 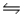
HSO3-(aq) + H2CO3(aq) .
Ka1(H2SO3) = 1 × 10-2; Ka1(H2CO3) = 4.2 × 10-7
Definitions:
Erotophobic
Refers to an irrational fear of or strong aversion to sexuality or sexual matters.
Masturbate
The act of self-stimulation of one's own genitals for the purpose of sexual pleasure.
Orgasm
The climax of sexual excitement, characterized by intensely pleasurable feelings centered in the genitals and, in some cases, other parts of the body.
Q1: The vapor pressure of water at 45.0
Q31: The K<sub>c</sub> for the reaction CO<sub>2</sub>(g)+ H<sub>2</sub>(g)
Q65: Which of the following liquids would make
Q72: How many grams of sucrose (C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>, 342.3
Q72: Consider a buffer solution prepared from
Q78: What is the osmotic pressure of a
Q80: A saturated sodium carbonate solution at 0°C
Q87: Which of the following is consistent
Q97: A reaction is experimentally found to follow
Q109: Consider a solution made from a nonvolatile