The equilibrium constant for the reaction C6H5COOH(aq) + CH3COO-(aq) 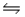
C6H5COO-(aq) + CH3COOH(aq)
Is 3.6 at 25°C.If Ka for CH3COOH is 1.8 × 10-5, what is the acid dissociation constant for C6H5COOH?
Definitions:
Vision-Impaired
A term describing individuals who have limited or no vision, encompassing a range of visual impairments from partial vision loss to total blindness.
Gazing Patterns
The way in which individuals look at others or objects in their environment, often studied to understand attention, attraction, and social cues.
Non-Verbal Communication
The process of conveying information or expressing feelings without the use of spoken words, through gestures, facial expressions, and body language.
Gaze
Looking at someone’s eyes.
Q20: Will H<sub>2</sub>(g)form when Sn is placed in
Q30: Hydrogen iodide decomposes according to the equation
Q33: When a solution of a certain gadolinium
Q68: At 10°C one volume of water dissolves
Q68: For the following reaction at equilibrium
Q86: Which of the following nuclear processes results
Q101: The data below were determined for
Q108: The normal freezing point of ammonia
Q135: Complete and balance the following redox
Q149: What is the pH of a 0.023