Predict the direction in which the equilibrium will lie for the reaction H3PO4(aq) + HSO4-(aq) 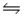
H2PO4-(aq) + H2SO4(aq) .
Ka1(H3PO4) = 7.5 × 10-3; Ka(H2SO4) = very large
Definitions:
Nutritious Organic
Describes food or substances derived from living matter that are beneficial to health due to their nutrient content.
Bacillus Anthracis
A bacterium that causes anthrax; it is rod-shaped and can be a serious pathogen affecting both humans and animals.
Legionella Pneumophila
A bacterium that causes Legionnaires' disease, a severe form of pneumonia, and is found naturally in freshwater environments.
Infectious Disease
Diseases caused by pathogenic microorganisms such as bacteria, viruses, parasites or fungi that can be spread, directly or indirectly, from one individual to another.
Q5: The equilibrium constant for the chemical equation<br>2NO(g)+
Q14: The equilibrium constant for the reaction C<sub>6</sub>H<sub>5</sub>COOH(aq)+
Q31: Which of the following aqueous solutions has
Q45: Aluminum does not corrode as does iron,
Q47: Identify the conjugate acid-base pairs in the
Q57: Find the temperature at which K<sub>p</sub>
Q66: What is the pH of a solution
Q67: A 0.14 M HNO<sub>2</sub> solution is 5.7%
Q105: In general, to calculate the rate constant
Q161: If the pH of pure water is