Calculate the equilibrium constant Kc for the following overall reaction:
AgCl(s)+ 2CN-(aq) 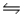
Ag(CN)2-(aq)+ Cl-(aq)
For AgCl, Ksp = 1.6 × 10-10; for Ag(CN)2-, Kf = 1.0 × 1021.
Definitions:
Intellectual Property
Legal rights which result from intellectual activity in the industrial, scientific, literary, and artistic fields, protecting creators' interests.
Privacy Laws
Privacy Laws are regulations designed to protect individuals' personal information and data from being misused, ensuring their privacy is respected and safeguarded.
Copyright
A legal right granted to an author, composer, playwright, publisher, or distributor to exclusive publication, production, or sale of their original works.
Whaling Attack
A whaling attack is a specialized form of phishing that targets high-profile individuals within an organization, like executives, with the intent to steal sensitive information or gain access to their networks.
Q10: What happens to nitrogen dioxide in sunlight?
Q47: Sulfur-35 decays by beta emission.The decay product
Q49: Which response includes all of the following
Q64: When a <sup>162</sup>Re nucleus emits an alpha
Q75: For the reaction at equilibrium: 3Fe(s)+ 4H<sub>2</sub>O(g)
Q83: The solubility of strontium carbonate is 0.0011
Q94: The half-life of <sup>90</sup>Sr is 29 years.What
Q96: The only stable isotope of fluorine is
Q101: If 12% of a certain radioisotope decays
Q150: Lime is used in farming to reduce