Multiple Choice
Calculate G° for the reaction 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) . 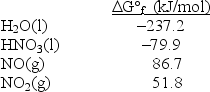
Definitions:
Related Questions
Q28: It is possible for the following
Q34: Sulfur can be separated from lead
Q47: Complete and balance the following redox
Q47: Calculate the molar solubility of silver carbonate
Q83: When the reaction 2H<sub>2</sub>S(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg" alt="When
Q94: If one starts with pure NO<sub>2</sub>(g)at a
Q95: Which one of the following equations represents
Q126: A tablet of a common over-the-counter drug
Q143: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 × 10<sup>-3</sup>,
Q148: Complete and balance the following redox