If one starts with pure NO2(g) at a pressure of 0.500 atm, the total pressure inside the reaction vessel when 2NO2(g) 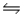 2NO(g) + O2(g) reaches equilibrium is 0.674 atm.Calculate the equilibrium partial pressure of NO2.
2NO(g) + O2(g) reaches equilibrium is 0.674 atm.Calculate the equilibrium partial pressure of NO2.
Definitions:
CRM Systems
Customer Relationship Management systems, technology platforms that help organizations manage and analyze customer interactions and data throughout the customer lifecycle.
Distribution Portals
Distribution portals are online platforms that allow businesses to distribute their products or services to a wide audience, often integrating various distribution channels.
Business Processes
Business Processes refer to a series of tasks or activities conducted by individuals or equipment that produce a specific service or product for a particular set of customers.
Customer Churn
The measure of how many customers stop using or subscribing to a service over a certain time period, often used to gauge a business's performance.
Q30: In general, to calculate the activation energy
Q35: Which of the following compounds is more
Q41: Identify the conjugate base of HCO<sub>3</sub><sup>-</sup><br>A)H<sub>2</sub>CO<sub>3</sub><br>B)CO<sub>3</sub><sup>2-</sup><br>C)OH<sup>-</sup><br>D)CO<sub>2</sub><br>E)CO
Q44: In which of the following would the
Q44: For the following reaction at equilibrium,
Q50: Calculate the silver ion concentration in a
Q67: Write an equation showing the net reaction
Q93: What mass of ammonium nitrate must be
Q96: Calculate the pH of a 0.021 M
Q116: Which of the following constants is/are