The equilibrium constant at 427°C for the reaction N2(g) + 3H2(g) 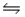 2NH3(g) is Kp = 9.4 × 10-5.Calculate the value of G° for the reaction under these conditions.
2NH3(g) is Kp = 9.4 × 10-5.Calculate the value of G° for the reaction under these conditions.
Definitions:
Supply Chain Responsiveness
The agility of a supply chain in responding to changes in demand, supply, and market conditions to maintain service levels and customer satisfaction.
Substitute Similar Products
Products that can be used in place of one another due to similarities in functionality, quality, or purpose.
Short Lead Times
The brief period between the initiation of a process and its completion, often associated with the efficiency of production or supply chain operations.
Coronal Section
A coronal section is an anatomical slice of the body or organ that divides it into front (anterior) and back (posterior) parts.
Q19: One of the steps in the
Q60: If 24% of a certain radioisotope decays
Q82: You have 500.0 mL of a buffer
Q85: What is the missing symbol in
Q93: Calculate the binding energy per nucleon of
Q93: Consider the following equilibrium,<br>4NH<sub>3</sub>(g)+ 3O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3246/.jpg"
Q96: Write the balanced overall redox equation for
Q97: Given the following data, calculate the boiling
Q127: Calculate the pH of the solution resulting
Q144: What is the pH of a 0.15