Find the temperature at which the reaction N2O4(g) 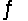 2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm.
2NO2(g) will be in equilibrium when both gases are present at partial pressures of 1.00 atm. 
Definitions:
Investment Center
A decentralized unit in which the manager has the responsibility and authority to make decisions that affect not only costs and revenues but also the fixed assets available to the center.
Authority
The power or right to make decisions, direct others, and enforce obedience within a particular scope.
Plant Assets
Fixed assets that are tangible in nature, such as buildings and machinery, used in the operations of a business and expected to be useful for many years.
Profit Margin
A measure of a company's profitability, typically defined as net income divided by revenue, and expressed as a percentage.
Q1: A possible reaction leading to removal
Q5: At 25 °C, the base ionization constant
Q24: A sample of solid naphthalene is introduced
Q30: An increase in the amount of particulate
Q35: Determine all of the covalent hydrides from
Q36: Decay of lutetium-167 by electron capture yields<br>A)ytterbium-167.<br>B)lutetium-166.<br>C)thulium-163.<br>D)tantalum-171.<br>E)hafnium-167.
Q58: List the given types of nuclear radiation
Q63: Calculate the pH of a 0.025 M
Q85: Consider this reaction at equilibrium at a
Q123: The solubility of Ba(NO<sub>3</sub>)<sub>2</sub> is 130.5 g/L