For the reaction CuS(s)+ H2(g) 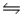
H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= - 20.6 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
Definitions:
Labor Costs
Expenses associated with employing labor, including wages, salaries, benefits, and taxes.
World Price
The international market price of a good, influenced by global supply and demand conditions.
Merchandise Trade
International trade in goods as opposed to services, including both exports and imports of physical products.
Tangible Products
Physical items that can be touched, seen, and measured, such as electronics, clothing, and furniture.
Q4: Write a net ionic equation for the
Q26: For the following reaction at equilibrium,
Q27: Starting with 0.250L of a buffer solution
Q34: The average person breathes about 20 m<sup>3</sup>
Q39: Consider the following standard reduction potentials in
Q56: Due to a highway accident, 150 L
Q60: A solution was prepared such that the
Q115: Which solution will have the lowest pH?<br>A)0.25
Q157: For H<sub>3</sub>PO<sub>4</sub>, K<sub>a1</sub> = 7.3 × 10<sup>-3</sup>,
Q168: Which of the following solutions is basic?<br>A)[H<sub>3</sub>O<sup>+</sup>]